Five months ago, I reported about data integrity concerns in 6 publications authored by Min-Jean Yin, who had been working at the pharma giant Pfizer in La Jolla, California, as Senior Principal Scientist since 2003. One paper, where she contributed as a collaborator (Lamoureux et al, European Urology, 2014), has been corrected already in March 2016. Five other cancer research papers, on the efficiency of Pfizer’s own pharmacological enzyme inhibitors, will now be retracted, after an investigation performed by Pfizer confirmed the suspicions of data manipulation, originally raised on PubPeer. These five papers stemmed directly from the Pfizer lab which Yin used to be in charge of. Used to be – because according to her recently updated LinkedIn profile, Yin doesn’t work there anymore. Since September 2016, she joined a rather unremarkable Californian biotech start-up Diagnologix LLC in San Diego, as “General Manager”. With such a career (and surely also salary) setback, it is safe to assume Yin did not leave Pfizer after 13 years of service entirely voluntarily.
This development stands in a stark contrast with another misconduct case with the German pharma giant and Pfizer’s market competitor, Boehringer Ingelheim. The company recruited the mitochondria biologist Tina Wenz as their “principal scientist” and “lab head”, just about when first accusations of data manipulations in her papers appeared. Wenz used to be a research group leader at the University of Cologne, her former employer found her guilty of misconduct and data manipulation and requested the retraction of six of her papers (see my report here). Boehringer however seems to have no issues with her questionable research style, since they declared to me repeatedly that her past academic research had no connection at all to what Wenz is currently doing for them. Who knows, maybe it was her special “creativity” which Boehringer’s recruiters were really after. In fact, the freshly acquisitioned Wenz still remains a proud leading member of the Boehringer research work force. Yin, on the other hand, had to depart from her long-established senior position at Pfizer in wake of the company’s investigation.
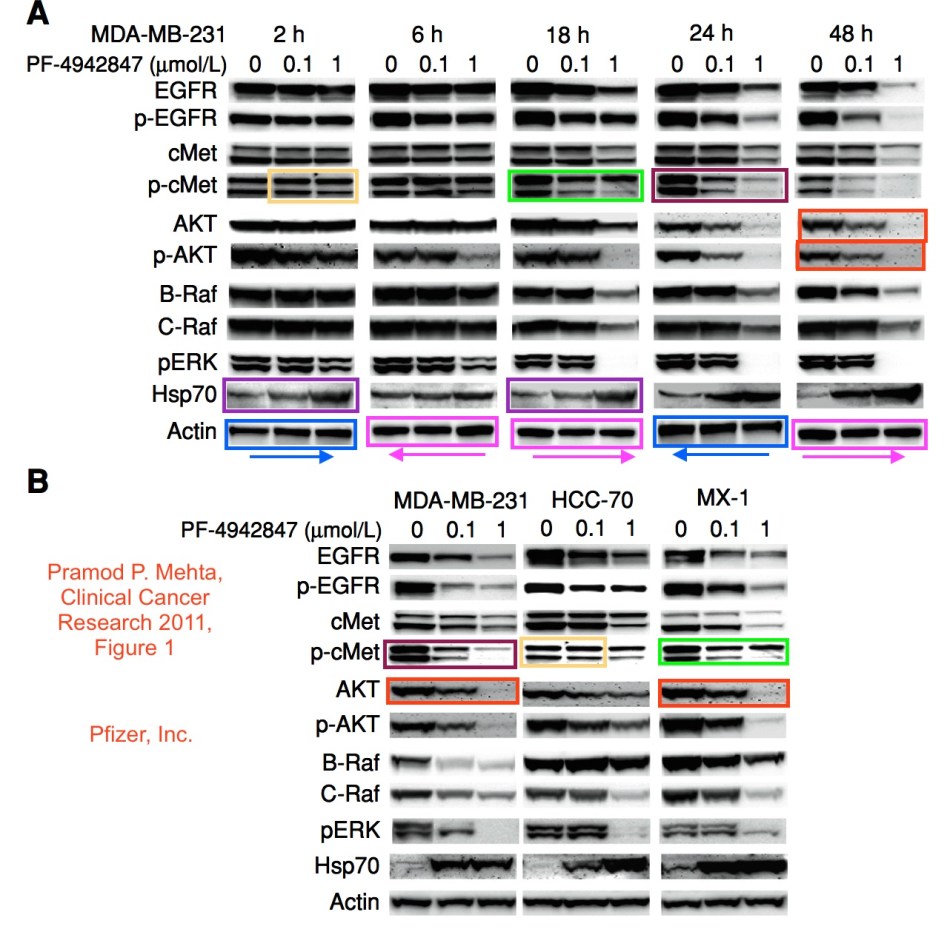
The Yin papers to be retracted are mentioned below. On October 10th 2016, Yvonne Cristovici, Assistant General Counsel in Compliance Division of Pfizer, wrote to me in regard to my Yin inquiry:
“In response to your concerns about possible duplicate images used in six journal articles associated in some way with the laboratory of a particular Pfizer researcher, we at Pfizer conducted a thorough investigation and write to provide you with an update.
The five papers that originated from that laboratory are:
Nassirpour et. al, miR-221 Promotes Tumorigenesis in Human Triple Negative Breast Cancer Cells, 8(4) PLOS ONE, (2013);
Baxi et. al, Targeting 3-Phosphoinoside-Dependent Kinase-1 to Inhibit Insulin-Like Growth Factor-I Induced AKT and p70 S6 Kinase Activation in Breast Cancer Cells, 7(10) PLOS ONE (2012);
Mehta et. al, A novel class of specific Hsp90 small molecule inhibitors demonstrate in vitro and in vivo anti-tumor activity in human melanoma cells, 300 Cancer Letters 30 (2011);
Mehta et. al, Effective Targeting of Triple-Negative Breast Cancer Cells by PF-4942847, a Novel Oral Inhibitor of Hsp 90, 17(6) Clinical Cancer Research 5432, 2011; and
Nassirpour et. al, Nek6 Mediates Human Cancer Cell Transformation And Is A Potential Cancer Therapeutic Target, 8(5) Molecular Cancer Research 717 (2010).
We have been able to confirm that all or nearly all of the images in these five articles that were flagged as potential duplicates on PubPeer.com indeed appear to be duplicates. Based on the findings from the investigation, Pfizer is recommending to the journals that all five articles be retracted, and Pfizer also has encouraged the first and corresponding/senior authors of each of the five papers to request that their article be retracted. The senior and corresponding author of each paper, Min-Jean Yin, Ph.D., has agreed with Pfizer’s recommendation to request retraction of each article. Each of the three scientists who served as first authors of these five papers, Pramod Mehta, Sangita Baxi, and Rounak Nassirpour, Ph.D., has also agreed to request retraction of the article or articles for which he or she served as first author. Pfizer has attempted to communicate with all remaining co-authors across the five papers to inform them of the investigative findings and has succeeded in reaching the vast majority of them. All co-authors who have responded to our attempts to contact them have concurred with the decision to seek a retraction of their article or articles.
As you may know, on the sixth publication you identified – Lamoureux et. al, Suppression of Heat Shock Protein 27 Using OGX-427 Induces Endoplasmic Reticulum Stress and Potentiates Heat Shock Protein 90 Inhibitors to Delay Castrate-resistant Prostate Cancer, 66 European Urology 145 (2014) – there was only one Pfizer scientist listed as an author, but for that paper, she was not the first author, or the corresponding author, or the senior author. The study was done primarily at the University of British Columbia. Therefore, we have been in contact with that institution regarding this allegation. We understand that a correction has been issued by the journal, at the request of the senior author from that university. It is available at the below weblink.
Thank you for raising this matter. Data integrity is paramount to Pfizer’s Research and Development efforts, and we appreciate your bringing this to our attention”
Update 11.10.2016: Cristovici also confirmed today:
“Dr. Yin is no longer employed at Pfizer”.

Pingback: Image integrity concerns in papers from a Pfizer lab – For Better Science
Pingback: Pfizer fires employee, requests five retractions - Retraction Watch at Retraction Watch
Pingback: Pfizer retracts 5 articles by top researcher who since left company | Awesome Investors
Pingback: Pfizer Retracts 5 Articles Authored by Top Cancer Researcher Who Has Since Left the Company - StockSocial
Pingback: October 13, 2016 | Microbiome Digest - Bik's Picks
Pingback: Pharmalot, Pharmalittle: New bill would create review board for drug prices – CRISPR
Pingback: Former Pfizer Cancer Scientist Gets All 5 Papers Retracted - StockSocial
Pharmaceutical companies should be the first ones in providing open access to original research raw data under certain rules in the near future
LikeLike
Pingback: Pfizer announces more retractions for sacked lab head Min-Jean Yin, whistleblower revealed – For Better Science